Bone Health in Cystic Fibrosis
CFReSHC CF-SRH Resource Guide by Patients for Providers and Patients
Key
For Providers
For Patients
For Patients and Providers
Introduction
Bone health is a critical concern in individuals with cystic fibrosis (CF) because there is an increased risk of osteoporosis and fractures associated with the disease.[1] Three factors characterize Cystic fibrosis-related bone disease (CFBD): 1) reduced bone mineral density; 2) a higher incidence of fractures; and 3) the development of kyphosis, which can limit lung expansion.[2]
Despite its importance, CFBD has received limited attention in research, partly due to patients’ previously low life expectancy.[1,3–5] This lack of attention has led to significant gaps in awareness of CFBD and its management among patients and providers.5 Researchers still need to delve into the various contributors to bone health in CF, including chronic inflammation, malabsorption of essential nutrients, and long-term corticosteroid use.[4,5] Beyond vitamin D deficiency and chronic inflammation, CFBD arises from a complex interplay of factors, including pancreatic insufficiency, vitamin K deficiency, CF-related diabetes, and hypogonadism.[3,4]
These challenges also contribute to pulmonary complications, like reduced lung function and a reduced ability to perform effective airway clearance techniques.1 Moreover, CFBD affects a growing number of aging individuals with CF: from as little as 5% in children to as high as 65% in adults over 45 years.[3] For their part, pediatric patients with CF have approximately half the measured bone mineral density (BMD) of their non-CF peers. Similar comparative rates occur in young adults, particularly relating to the spine and distal radius.[3] Alarmingly, these findings have persisted for decades, even with lung function improvements and vitamin D supplementation to treat deficiency.[3]
Emerging therapies, such as cystic fibrosis transmembrane conductance regulator (CFTR) modulators, offer hope for improving bone health outcomes in patients with CF.[6] If introduced to patients with CF at younger ages, these therapies may mitigate the risk of developing bone disease later in life. Large, long-term studies are needed to more deeply understand the underlying mechanisms of CFBD and to evaluate the broader clinical implications of elexacaftor/tezacaftor/ivacaftor (ETI) on bone health.[1,6]
To manage CF-related bone disease effectively, doctors should ask their patients:
- Have you experienced any fractures? If yes, what was the mechanism?
- Have you had any joint pain in the past year?
- Are you currently taking any vitamin D or calcium supplements?
- Do you participate in weight-bearing exercise regularly?
- Have you had a recent bone density scan?
- If you have osteoporosis, are you experiencing any new or worsening symptoms of depression or anxiety?
- Have you ever been on systemic corticosteroid treatments?
Patients with CF should consider asking their healthcare provider:
- What is my current bone density, and how does it compare to normal levels? How does it compare to my last DXA?
- What steps can I take to improve my bone health?
- Are there any medications or supplements I should take to prevent bone loss?
- How often should I get a bone density test?
- What are my treatment options for managing osteoporosis?
- What impact do modulator therapies have on bone health?
Causes of Cystic Fibrosis-Related Bone Disease
CFBD is a multifactorial condition caused by the complex interplay of nutritional deficiencies and the effects of chronic inflammation. The dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) protein primarily drives these issues. CFBD involves both direct and indirect mechanisms affecting bone metabolism.
Bone Physiology
Bone mass: Individuals achieve peak bone mass in early adulthood. Many factors influence the accrual of bone, including nutrition, physical activity, biological sex, and timing of puberty.[7]
Cortical and Trabecular bone: Bone consists of a dense outer layer (cortical bone) and an inner structure of plates and rods (trabecular bone). The vertebrae and the ends of long bones are mainly trabecular bone. Long bone shafts are largely cortical bone. When excess bone resorption occurs, bone minerals are lost more rapidly in the trabecular bone. Imbalances in bone remodeling can increase the risk of fractures.[7]
Bone metabolism: Continuous bone remodeling maintains skeletal strength and mineral balance. During remodeling, osteoblasts (which build new bone) and osteoclasts (which reabsorb old bone) synchronize. Osteocytes are former osteoblasts embedded in the skeletal matrix. Osteocytes act as mechanical sensors and coordinate osteoblast and osteoclast activity. Inflammatory hormones (cytokines), insulin-like growth factors, and bone morphogenic proteins also play essential roles in managing the production and activity of the bone cells.[7]
Direct Effects of CFTR Dysfunction
Osteoblasts, osteoclasts, and osteocytes express CFTR; thus, CFTR deficiency is associated with impaired osteoblast activity and differentiation. It is also associated with an increase in the number and activity of osteoclasts, which leads to bone degradation. Studies have shown that CFTR deficiency also leads to the overexpression of nuclear factor kappa-B ligand (RANKL) and the production of defective osteoprotegerin (OPG), both critical regulators of bone turnover.[1]
Chronic Inflammation
Chronic pulmonary infections in CF patients lead to systemic inflammation. Elevated pro-inflammatory cytokine hormones characterize this outcome. These cytokines enhance bone resorption (which induces osteoclast activity) and inhibit bone formation by impairing osteoblast formation. The process further exacerbates bone loss and increases the risk of fractures. Systemic inflammation in CF can directly interfere with the bone remodeling process, which can lead to lower bone mineral density over time.[1]
Nutritional Deficiencies
About 90% of people with CF (PwCF) have exocrine pancreatic insufficiency. Pancreatic insufficiency in CF patients results in the malabsorption of fat-soluble vitamins (A, D, E, and K). CF-related diabetes, poor appetite, pain with swallowing, and some medications can also cause nutritional issues.
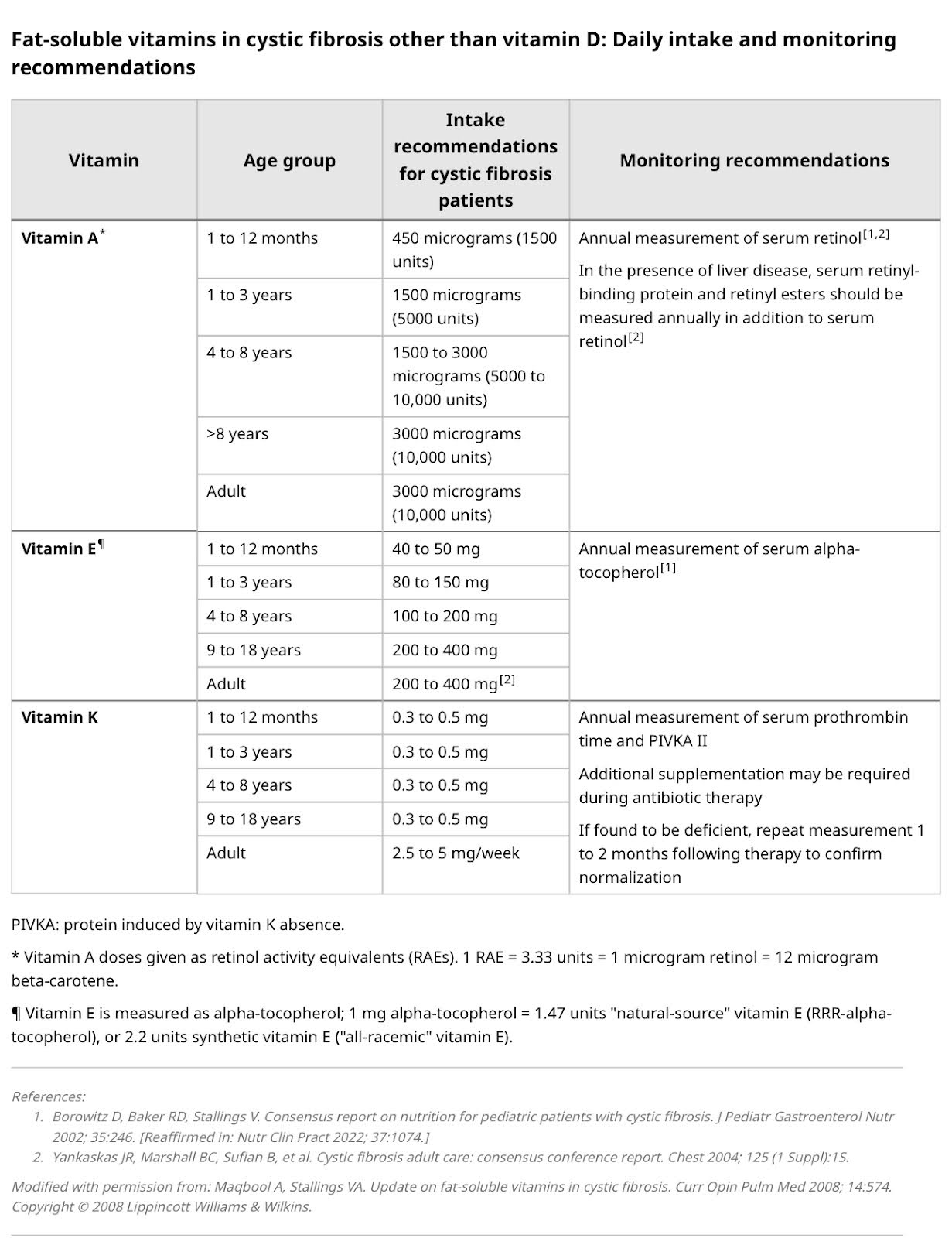
Vitamins A, D, E, and K are crucial for bone health. Vitamin D regulates calcium and phosphorus levels and is essential for intestinal calcium absorption. Vitamin K also plays a key role in bone formation. Deficiencies in these vitamins impair bone mineralization and contribute to reduced bone density.[7,8]
Physical Inactivity
CF patients often experience reduced physical activity due to chronic lung disease, which can negatively impact bone health. Physical inactivity reduces the mechanical stress on bones, leading to decreased bone formation. Even for individuals who do not have CF, decreased activity can be associated with lower bone density.[4,7,8]
Glucocorticoid Therapy
The long-term use of glucocorticoids (steroid medications), commonly used by CF patients to treat pulmonary exacerbations, increases bone resorption and decreases bone formation. Glucocorticoids can also impair intestinal and renal calcium absorption, decrease sex and growth hormone production, and induce high blood sugars. These actions significantly contribute to the development of osteoporosis and increased fracture risk in CF patients.[4,7]
Altered Sex Hormone Production
Altered sex hormone production affects peak bone mass accumulation, particularly when occurring during puberty. It can lead to lower bone density in adulthood. Puberty is a critical period for bone development, and disruptions can have long-term consequences on bone health. Though pubertal delay was previously documented in 20-60% of PwCF, recent studies demonstrate no difference in the timing of puberty. Individuals who have pubertal delay accrue their peak bone mass late and have lower bone density at age 25 compared to their healthy peers.[9]
In summary, CFBD is caused by a combination of direct effects of CFTR dysfunction on bone cells, chronic inflammation, malnutrition, physical inactivity, glucocorticoid therapy, and altered sex hormone production. Understanding these mechanisms is crucial for developing effective therapeutic strategies to manage and treat CFBD.[3]
Testing for CF-Related Bone Disease
Laboratory Tests
Blood tests can assess the nutritional status of CF patients and identify any risks for bone disease. Measuring serum calcium, vitamin D, phosphate, and parathyroid hormone (PTH) levels is standard. Low level of vitamin D are common in PwCF, especially for those who have pancreatic insufficiency. US guidelines recommend that patients have their vitamin D levels taken annually and repeat testing if vitamin dosing changes. European guidelines support additional, periodic measurements of calcium, phosphorus, PTH, and urine calcium levels.[9]
If routine screening detects osteoporosis or a rapid decline in bone density, a physician should order labs to rule out other causes of osteoporosis. These labs may include a complete blood count, blood chemistry, liver enzymes, vitamin D levels, alkaline phosphatase, parathyroid hormone, 24-hour urine calcium, and sex hormone levels. Bone turnover markers may also be tested, though there are no studies that have validated these markers in CFBD.[7]
Bone Mineral Density (BMD) Testing
Guidelines recommend that a patient’s bone density be screened for all adults 18 years and older with CF. Children who are at least 8 years old may need screening if they have low body weight, markedly abnormal pulmonary function tests, have been exposed to steroid medications for more than 90 days per year, and have delayed puberty or fractures.[3]
The gold standard for diagnosing CFBD is a bone mineral density (BMD) test, typically performed using dual-energy X-ray absorptiometry (DXA). DXA scans measure bone density in key areas like the spine, hip, and wrist, as they are most vulnerable to fractures. Ratti et al. showed that CF patients’ BMD screening rates are lower than recommended; an average of 60-66% of patients get a DXA scan, despite the fact that early detection is crucial to prevent fractures and manage bone health. [7,10,11]
Bone Density Testing Methods
There are six methods to measure bone density.
1. Dual-Energy X-ray Absorptiometry (DXA): Clinicians consider the DXA to be the gold standard to assess bone mineral density (BMD) in CF patients. Its advantages over other methods include widespread availability, minimal radiation exposure, low cost, and a high level of reproducibility. DXA measures energy absorption to determine bone mineral content and the bone area of a selected site.
In adults, results are reported as a T score that indicates how much the bone density differs from that of a young healthy person at peak bone mass (25-30 years of age). A Z score is used in children, adolescents, and young adults. The Z score describes how a patient’s bone density differs from an age and sex matched reference group. DXA results may need to be adjusted based on the patient’s height and timing of puberty .[7,12]
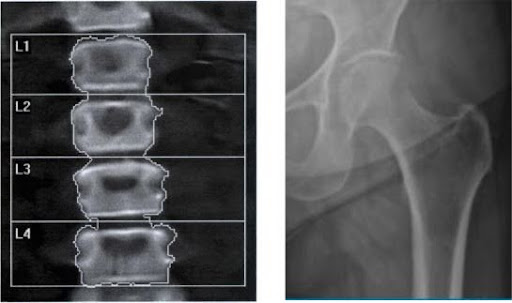
Figure 1. Representative lumbar spine (left) and hip (right) DXA.12
2. Trabecular Bone Score (TBS): TBS is a tool complementary to the DXA derived from lumbar spine DXA results. It looks at the architecture of the lumbar spine and has been shown to enhance fracture prediction in other high-risk groups, like patients on chronic steroids.[7]
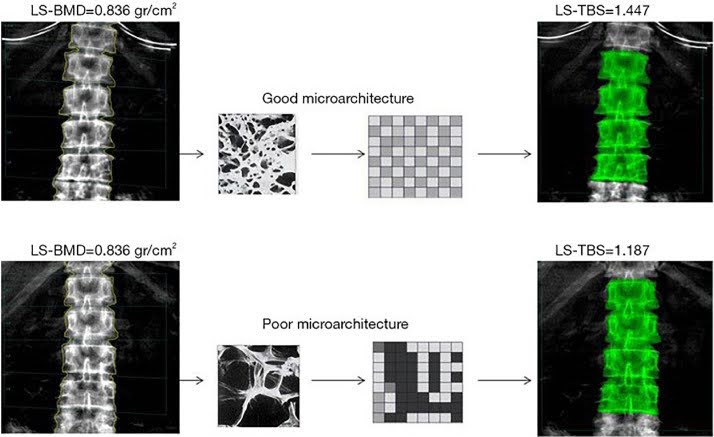
Figure 2. DXA images of the spine, L1–L4 level of two individuals (top and bottom row); LS-BMD values are the same for both, LS-TBS in the second subject is lower compared to the first subject, corresponding to deteriorated microarchitecture of the vertebral body.[7]
3. Fracture risk assessment tool (FRAX®): FRAX® is an online calculator that predicts the 10-year probability of major osteoporotic fractures and hip fractures using risk factors and femoral neck bone mineral density. It does not account for spinal changes. TBS can be applied to FRAX® to enhance the prediction of fracture risk.[7]
A link to FRAX® is provided here: https://frax.shef.ac.uk/FRAX/tool.aspx?country=9
4. Quantitative Ultrasound (QUS): QUS can complement DXA at peripheral sites of the body, like the heel (calcaneus), lower arm (radius), lower leg (tibia), and the toes (phalanges). QUS is inexpensive, portable, and has no radiation exposure. It measures how the bones absorb sound waves. However, it has a lower detection rate of low bone density than DXA, so it is less reliable as a standalone diagnostic tool. Though QUS has been validated for detecting normal bone density in populations with a low chance of fracture, it is not used for the diagnosis of or as a monitoring method for osteoporosis.[7,12]
5. Quantitative Computed Tomography (QCT): QCT offers three-dimensional imaging and can assess bone density and structure. It can also determine the difference between trabecular and cortical bone. Peripheral QCT (pQCT) can detect differences in bone density and strength at peripheral sites, like the radius. As such, it may be useful in pediatric populations. It is costly and can expose patients to radiation, so it is only used in research settings.[7,12]
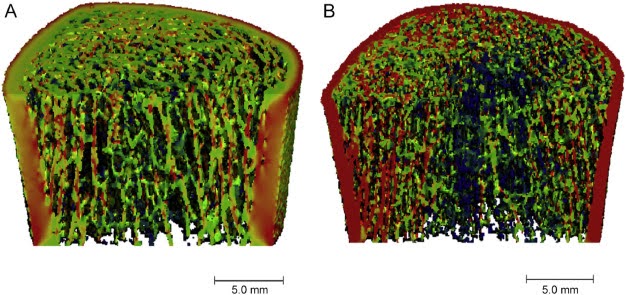
Figure 3. Representative HR-pQCT images of the tibia of a 23-year-old healthy woman (A) and a 23-year-old woman with CF (B)[7,12]
6. Magnetic Resonance Imaging (MRI): MRIs are used to detect vertebral body abnormalities and fractures that X-rays and DXAs cannot. An MRI can distinguish between trabecular and cortical bone and can evaluate the bones’ microarchitecture. It is time-consuming and expensive, and is not yet validated for the diagnosis or monitoring of osteoporosis.[7,12]
DXA remains the gold standard test to measure bone density. Other imaging options can complement the DXA or can be used for research. Guidelines recommend repeated bone density measurements based on T-score results and a patient’s overall clinical scenario. Regular monitoring and early detection of bone density issues are essential for effectively managing CFBD.
Treatments for CFBD
Treatment of CFBD requires a comprehensive approach, including nutritional support, lifestyle modifications, and medications.
Nutritional Support
A well-balanced diet rich in calcium and vitamin D is essential for individuals with CF to maintain their bone health. Since CF patients often have difficulties absorbing nutrients, it is critical to ensure they consume sufficient amounts of calcium and vitamin D. Dairy products, leafy greens, fortified cereals, and fish are excellent sources of calcium and vitamin D. Patients with pancreatic insufficiency should use pancreatic enzymes to reach their nutritional goals.
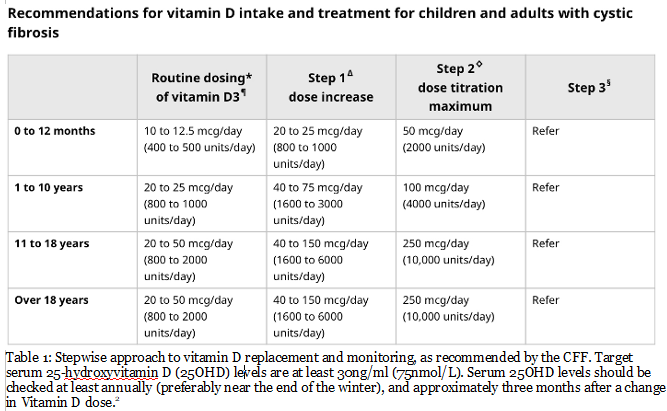
Vitamin D and Calcium Supplementation: Besides diet, many individuals with CF may need to supplement their dietary calcium and vitamin D intake of meet their nutritional requirements and maintain their bone health.[9,13] Though recommended vitamin D and calcium supplementation is the same for the CF population as it is for the general population, physicians may need to adjust doses according to the levels measured during routine laboratory screening.[2,9,13] Juhász et al. found, for instance, that vitamin D supplementation significantly improves levels of vitamin D in PwCF.[14]
Lifestyle Modifications
Physical Activity: Regular weight-bearing exercise plays a key role in maintaining bone strength and reducing fracture risk for people with CF. Regular exercise, like walking, running, or resistance training, is also beneficial because it helps stimulate the formation of bone during a patient’s development and in adulthood. Exercise programs should be tailored to the individual’s capabilities and health status.[9,15]
Minimizing Risk Factors: Patients should minimize their use of glucocorticoids and other medications that negatively impact bone health. Avoiding a sedentary lifestyle can benefit long-term bone health.[1,9]
Pharmacologic Treatments
Bisphosphonates: These medications are the mainstay of CFBD treatment. Bisphosphonates like alendronate and risedronate inhibit bone resorption by impeding osteoclast activity. Researchers have shown that bisphosphonates increase bone mineral density (BMD) in CF patients. Bisphosphonates are particularly beneficial in the lumbar spine and hips. Still, researchers do not fully understand the long-term effects of bisphosphonates on fracture risk in CF patients, so careful monitoring is necessary.[9,16]
CFTR Modulators: Modulator therapy, like Ivacaftor, has the potential to improve bone density and microarchitecture in patients with the G551D-CFTR mutation. Putman et al. demonstrated that there are significant increases in cortical volume, area, and porosity at the radius and tibia in adults treated with ivacaftor, which suggests there are benefits of modulators for bone health.[6] A more detailed discussion of modulator therapy and bone health follows.
Monoclonal Antibodies: Emerging treatments, such as denosumab and romosozumab, target bone resorption and formation pathways, respectively. Though denosumab decreases osteoclast activity, treatment with another agent must follow its use to avoid rebound vertebral fractures. Romozomab increases bone formation and decreases bone resorption, but its use is limited to 12 months in patients with a low risk for cardiovascular disease. [9]
Anabolic Agents: Teriparatide and abaloparatide, which stimulate bone formation, are also considered as possible treatments for CFBD . They are parathyroid hormone analogs and activate osteoblasts, thus promoting new bone formation. These treatments may be beneficial for patients with severe osteoporosis, but further investigation is needed to determine efficacy in CF.[9]
Calcitonin: Calcitonin is a nasal spray used in postmenopausal women who cannot use other treatments. Yet researchers have not determined its effectiveness in preventing fractures.[9]
Estrogen Receptor Modulators: Selective Estrogen Receptor Modulators (SERM), like Raloxifene and Bazedoxifene, are approved to prevent osteoporosis in postmenopausal females. Doctors prescribe Raloxifene for treatment, and use Bazedoxifene in combination with estrogen to treat menopausal symptoms.
Menopausal Hormone Therapy (MHT): Helps bone health in the late perimenopausal and early postmenopause stages. Researchers are investigating the use of MHT for the treatment of bone disease. Its use is currently limited to postmenopausal females with CF.[9]
CFBD Management: Combines pharmacologic treatments, nutritional support, and lifestyle modifications. Bisphosphonates remain the cornerstone of therapy, while CFTR modulators and emerging treatments offer additional options. Ensuring adequate vitamin D and calcium intake, optimizing nutrition, and promoting weight-bearing physical activity are essential components of a comprehensive treatment plan.[2,9]
Mental Health
Mental health is a critical aspect of care in individuals with cystic fibrosis (CF), particularly for those with cystic fibrosis-related bone disease (CFBD).[10] The physical challenges of CFBD, compounded by the emotional and social burdens of living with a chronic condition, contribute to an elevated risk of mental health concerns, including anxiety and depression.[10] Osteoporosis, a common complication in CFBD, has been linked to depression.[8]
The psychological consequences of chronic illnesses like CFBD arise from the interplay between physical, emotional, and social limitations.[8,10] Osteoporosis, for example, is associated with reduced mobility, chronic pain, and difficulties in performing daily activities, all of which can heighten feelings of frustration, hopelessness, and emotional distress.[17] These limitations may lead to increased dependency on caregivers and may alter social interactions, further diminishing quality of life. The CF care team should offer counseling to individuals with cystic fibrosis who are newly diagnosed with bone disease.[10]
Researchers have associated depression with physiological changes that negatively affect bone health.[10] For instance, they have found that elevated cortisol levels, associated with chronic stress and depression, can accelerate bone loss. Reduced sex hormone levels in individuals with CF can also promote the decline of bone mineral density.[7–9] Such findings highlight the intricate relationship between mental and physical health and underscore the importance of using approaches that integrate clinical and psychological care to improve outcomes for patients with CFBD.[8,10]
CFBD and CF Modulators
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have transformed CF care; they have enhanced patients’ pulmonary function, nutritional status, and overall survival.[1,9] Yet, knowledge about their effects on CFBD is still evolving.[6,20] Chronic inflammation, malabsorption, and long-term corticosteroid use cause CFBD, resulting in decreased bone mineral density (BMD), increased fragility fractures,[18] and skeletal abnormalities, such as kyphosis.[2] To optimize patients’ long-term musculoskeletal health, we must understand how CFTR modulators influence these mechanisms is essential.[2,9,18]
Emerging research suggests that CFTR modulators may improve bone health via multiple pathways.[1,9] By enhancing pancreatic function and nutrient absorption, modulators may improve calcium and vitamin D metabolism by enhancing pancreatic function and nutrient absorption. The latter are key to maintaining skeletal integrity.[2] Reductions in systemic inflammation and overall health improvements could also promote better bone remodeling.[1]
CFTR modulators may also directly affect bone cells, particularly osteoblasts; dysfunctional CFTR in osteoblasts may impair bone formation.[9] Early clinical data indicate that modulators help increase BMD and reduce fracture risk, though long-term studies are needed to confirm these benefits.[1,9,19,20]
Modulators may promote bone health by addressing underlying factors that contribute to CFBD.9,19 Researchers have shown that reducing inflammation can decrease bone resorption.[1] They have also shown that improved endocrine function, including attaining normal sex hormone levels, may enhance bone formation.[9] Studies are currently looking at the direct role of CFTR activity in regulating the function of osteoblasts and osteoclasts to offer insights into its benefits on bone health.[1]
Despite some promising research findings, critical knowledge gaps still remain regarding the effects of modulators on bone health.[9] We need long-term studies to determine whether the early benefits of modulators translate into sustained reductions in fracture risk and in the prevalence of osteoporosis in CF.[19] In addition, to optimize bone health in CF and prevent CFBD, patients will require additional approaches, like tailored nutritional support, weight-bearing exercise, and targeted pharmacologic interventions such as vitamin D supplementation,[14,20] bisphosphonates, monoclonal antibodies, and selective estrogen receptor modulators (SERMs).[1,9,15,19]
While CFTR modulators may improve bone health for individuals with CF, further research is needed to describe their role in preventing CF-related bone disease.[6,9,19] Ongoing studies will hopefully define the long-term impact of modulators and how they impact musculoskeletal outcomes.
Lung Transplant and Bone Health Relationship
The interplay of CFBD and lung transplantation is a complex phenomenon because both CF progression and transplantation introduce challenges to optimal bone health. While lung transplantation improves respiratory function and extends life expectancy, it can also lead to new risks for bone disease.[4]
Pre-existing bone fragility is a critical concern for these patients, as poor bone health before transplant increases the likelihood of complications, including intraoperative fractures and prolonged post-surgical recovery.[18,21] Osteoporosis is commonly diagnosed in pre-transplant patients, with fragility fractures occurring in up to 50–60% of cases.[9,18] Given the mechanical stresses associated with surgery and the systemic impact of CF, weakened bone structure can amplify fracture risk during and after transplant.
Emerging research suggests that bone health may improve post-transplant, with some studies reporting a reduction in fragility fractures and the stabilization of bone mineral density (BMD).[21] Despite these findings, studies on long-term bone health in transplanted individuals with CF remain limited.[16] There is a growing consensus among clinicians that proactive strategies to prevent and manage CFBD should be a priority before and after transplantation, and that researchers should study the long-term trajectory of bone health in post-transplant patients.[9,21,22]
The combined effects of immunosuppressive therapy and reduced physical activity post-transplant can further exacerbate bone loss. This can perpetuate a cycle of progressive skeletal deterioration.[9,16,18] High-dose corticosteroids, calcineurin inhibitors, and anti-metabolite medications are critical for preventing organ rejection but have adverse effects on bone metabolism, including accelerating bone resorption, impairing bone formation, and contributing to osteoporosis.[16,18,21] These processes significantly increase fracture risk post-transplant.[16]
Medication Management: There are currently no definitive guidelines for managing bone loss before and after lung transplantation.[18] Yet, patients must balance their need for immunosuppressive therapy with strategies to protect their bone health. Bisphosphonates, vitamin D analogs, and other osteoporosis treatments may help mitigate bone loss in high-risk patients.[18] Individuals should ingest adequate amounts of calcium, vitamin D, and other essential nutrients to maintain their bone health.[15] Given the malabsorption challenges in CF, dietitians should develop tailored nutritional strategies to optimize bone density while accommodating any dietary restrictions.
Studies indicate that introducing bone-active treatments early on after transplant can reduce fractures and stabilize bone mineral density (BMD).[18,21] These findings highlight the need for doctors to manage bone health proactively in routine post-transplant care.
Physical Therapy and Exercise: Post-transplant recovery period can involve prolonged inactivity, which can weaken patients’ bone structure, and increase fracture risk.[2,18,19,22] Performing weight-bearing and resistance exercises can help maintain bone strength and prevent further deterioration.[2,17] Physical therapists associated with care teams should develop individualized exercise programs that consider the patient’s pulmonary status and physical limitations but also promote long-term musculoskeletal health.
Mental Health Considerations: The mental health challenges of having CFBD and being transplanted should not be overlooked. Vertebral fractures and advanced CFBD can impact transplant eligibility, adding significant emotional distress for patients.[12] Further, the fear of fractures and long-term complications can contribute to anxiety and depression.[8] Mental health professionals can provide coping strategies, emotional support, and interventions to promote patients’ overall well-being throughout the transplant process.
Summary
CF and transplant teams can address the multifaceted challenges of CFBD by integrating targeted medication strategies, nutritional support, physical rehabilitation, and mental health care. A comprehensive, multidisciplinary approach can optimize post-transplant outcomes and ensure long-term bone health in individuals with CF.
Post-transplant medications and a sedentary lifestyle can worsen pre-existing CFBD and lead to a vicious cycle of declining bone health.[9,16,18] Immunosuppressive therapies, like high-dose corticosteroids, calcineurin inhibitors, and anti-metabolite medications, are essential for preventing organ rejection but adversely affect bone remodeling.[16,18,21] They accelerate bone resorption, reduce bone formation, and contribute to osteoporosis.[16]
As such, physicians must adopt a comprehensive, multidisciplinary approach to address the interplay of CFBD and lung transplantation. Educating patients on the impact of bone health, the importance of treatment adherence, and lifestyle modifications empowers them to take an active role in their care. By proactively managing CFBD, CF and transplant care teams can improve surgical outcomes, enhance recovery, and ultimately improve patients’ long-term quality of life.
CFBD and Future Research
While researchers have made significant progress in understanding CF-related bone disease (CFBD), there is still a need to study how to improve diagnostic tools and therapeutic strategies. Several ongoing studies are actively recruiting participants to explore CFTR modulators and osteoporosis.
One such study, led by Dr. Erik Imel at Indiana University, is investigating the direct and indirect effects of CFTR modulators on bone and muscle. This research examines potential cellular impacts on bone and muscle tissue and secondary benefits like improved nutrition, increased physical activity, and enhanced lung function. [https://research-studies.allinforhealth.info/us/en/listing/10806/cftr-modulator-effects-on/]
Additionally, researchers at the University of Texas are conducting a study to identify specific biomarkers associated with osteoporosis in individuals with CF. This study seeks to improve our knowledge of bone density issues in CF to lead to earlier detection and targeted interventions. [https://clinicaltrial.be/en/details/64112?only_active=0&only_eligible=0&only_recruiting=0&per_page=100&utm]
Further research is particularly needed in children and young adults because most CFBD studies focus on participants over 18 years of age.[14] Exploring how to improve assessment tools for fracture risk, bone mineral density measurements, and advanced imaging techniques would help promote early detection and intervention strategies. Assessing the long-term effects of CFTR modulators on bone health is also needed.[1]
Peer to Peer Advice
- Check with your CF clinic about taking Calcium, Vitamin D, and Vitamin K.
These are essential for bone health, but absorption can be challenging with CF. - Take your ADEK vitamins and pancreatic enzymes to support proper nutrient absorption.
- Stay active. Follow your doctor’s guidance on what activities are safe for you. When possible, include weight-bearing exercises like resistance training or light weight lifting to help strengthen your bones.
- Support your posture and balance.
If you’re able, try exercises that improve posture and core strength. CF can sometimes negatively affect posture due to chronic coughing or muscle imbalances, impacting bone health and stability. - Talk to your doctor about bone density testing, and ask if you qualify for early screening. Long-term steroid use (common in CF and post-transplant) can significantly reduce bone density over time.
- Avoid smoking and limit alcohol intake.
Both can gradually weaken your bones. Cutting back or eliminating these can make a big difference in long-term bone strength.
Resource Links: More Information about Osteoporosis
American Association of Clinical Endocrinology
Osteoporosis Patient Information
https://www.aace.com/disease-and-conditions/osteoporosis/all-about-osteoporosis
Dietary Guidelines for Americans
Food Sources of Select Nutrients
WebMD
Exercise for Strong Bones
https://www.webmd.com/osteoporosis/bone-strength-exercises
International Osteoporosis Foundation
This guide is available in several languages
”That’s Osteoporosis.”
World Health Organization FRAX tool
Fracture risk assessment tool
https://frax.shef.ac.uk/FRAX/tool.aspx?country=9
Cystic Fibrosis Foundation Bone Disease in CF Clinical Care Guidelines
https://www.cff.org/medical-professionals/bone-disease-cf-clinical-care-guidelines
Works Cited
- Fonseca Ó, Gomes MS, Amorim MA, Gomes AC. Cystic Fibrosis Bone Disease: The Interplay between CFTR Dysfunction and Chronic Inflammation. Biomol Basel, Switzerland. 2023;13(3):425. doi:10.3390/biom13030425
2. Baker S, Stallings V, Baker R. Cystic Fibrosis: Nutritional Issues. uptodate.com. Published online November 15, 2023. Accessed November 17, 2024. uptodate.com
3. Ticona JH, Lapinel N, Wang J. Future Comorbidities in an Aging Cystic Fibrosis Population. Life Basel, Switzerland. 2023;13(6):1305. doi:10.3390/life13061305
4. Dickinson KM, Collaco JM. Cystic Fibrosis. Pediatr Rev. 2021;42(2):55-67. doi:10.1542/pir.2019-0212
5. Aris RM, Merkel PA, Joseph PM, et al. Consensus statement : Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90(3):1888-1896. doi:10.1210/jc.2004-1629
6. Putman MS, Greenblatt LB, Bruce M, et al. The Effects of Ivacaftor on Bone Density and Microarchitecture in Children and Adults with Cystic Fibrosis. J Clin Endocrinol Metab. 2021;106(3):e1248-e1261. doi:10.1210/clinem/dgaa890
7. Anabtawi A, Le T, Putman M, Tangpricha V, Bianchi ML. Cystic fibrosis bone disease: Pathophysiology, assessment, and prognostic implications. J Cyst Fibros. 2019;18:S48-S55. doi:10.1016/j.jcf.2019.08.018
8. Kashfi SS, Abdollahi G, Hassanzadeh J, Mokarami H, Khani Jeihooni A. The relationship between osteoporosis and depression. Sci Rep. 2022;12(1):11177-11177. doi:10.1038/s41598-022-15248-w
9. Ullal J, Kutney K, Williams KM, Weber DR. Treatment of cystic fibrosis-related bone disease. J Clin Transl Endocrinol. 2022;27:100291-100291. doi:10.1016/j.jcte.2021.100291
10. Burgel PR, Southern KW, Addy C, et al. Standards for the care of people with cystic fibrosis (CF): recognising and addressing CF health issues. J Cyst Fibros. 2024;23(2):187-202. doi:10.1016/j.jcf.2024.01.005
11. Ratti GA, Fernandez GS, Schechter MS, et al. Bone mineral density screening by DXA for people with cystic fibrosis: A registry analysis of patient and program factors influencing rates of screening. J Cyst Fibros. 2022;21(5):784-791. doi:10.1016/j.jcf.2022.01.011
12. Williams K, Darukhanavala A, Hicks R, Kelly A. An update on methods for assessing bone quality and health in Cystic Fibrosis. J Clin Transl Endocrinol. 2022;27:100281.
13. Frantzen T, Barsky S, LaVecchia G, Marowitz M, Wang J. Evolving Nutritional Needs in Cystic Fibrosis. Life Basel, Switzerland. 2023;13(7):1431. doi:10.3390/life13071431
14. Juhász MF, Varannai O, Németh D, et al. Vitamin D supplementation in patients with cystic fibrosis: A systematic review and meta-analysis. J Cyst Fibros. 2021;20(5):729-736. doi:10.1016/j.jcf.2020.12.008
15. Jad R, Ma X, Stanojevic S, et al. Longitudinal changes in BMD in adults with cystic fibrosis. J Bone Miner Res. 2024;39(12):1716-1721. doi:10.1093/jbmr/zjae139
16. Conwell LS, Jeffery TC, Chang AB, Conwell LS. Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database Syst Rev. 2023;2023(1):CD002010. doi:10.1002/14651858.CD002010.pub5
17. NIH. Bone Health and Osteoporosis. Accessed September 5, 2025. https://www.niams.nih.gov/health-topics/bone-health-and-osteoporosis
18. Grassi G, Cairoli E, Gentile LMS, et al. Bone Disease in Long-Term Lung Transplant Survivors. Life Basel, Switzerland. 2023;13(4):928. doi:10.3390/life13040928
19. Gur M, Bar–Yoseph R, Hanna M, et al. Effect of Trikafta on bone density, body composition, and exercise capacity in CF: A pilot study. Pediatr Pulmonol. 2023;58(2):577-584. doi:10.1002/ppul.26243
20. Mon N, Krishnasamy SS, Bakeerathan G, Healy L, Favier E, Foushee S. 7522 Effects of Trikafta On Bone Mineral Density And Body Composition In Patients With Cystic Fibrosis. J Endocr Soc. 2024;8(Supplement_1). doi:10.1210/jendso/bvae163.413
21. Durette G, Jomphe V, Bureau NJ, et al. Long-term bone mineral density changes and fractures in lung transplant recipients with cystic fibrosis. J Cyst Fibros. 2021;20(3):2123-2123. doi:10.1016/j.jcf.2020.09.012
22. Tran TVM, Li X, Maalouf NM. Bone health outcomes in post-lung transplant patients with cystic fibrosis. J Cyst Fibros. 2023;22(3):381-387. doi:10.1016/j.jcf.2023.01.003
Image: Accessed August 18, 2025: https://unsplash.com/s/photos/bone-health
Free Printable PDF Download
Want a free printable PDF download of this section for your use in clinic? Just give us your name and email address below to get your download link. This will not add you to our email list.
