Contraception
any method, medicine, or device used to prevent pregnancyCFReSHC CF-SRH Resource Guide by Patients for Providers and Patients
In this section:
- Introduction
- Priority Questions
- Unique Concerns
- Contraception Counseling
- Myths and Facts
- CF-Specific Considerations
- Hormonal Contraception
- Other Reasons to take Contraception
- Post-Transplant
- CFTR Modulators
- Psychosocial
- Further Research
- Peer-to-Peer Advice
- Resource Links
- Works Cited
Key
For Providers
For Patients
For Patients and Providers
Introduction
Women with CF can get pregnant. However, the decision to become pregnant carries special considerations for women with CF [see the Pregnancy and Family Building chapters in this guide for more information]. As such, it is important for those who are sexually active but not actively trying to become pregnant to consider: a) using contraception; and b) which contraceptive method is best for them.
This choice is theirs alone to make, but discussions with a women’s health provider, CF provider, and in some cases, the patient’s partner, can help inform the decision.
- Do you want to get pregnant within the next year? During the next 5 years?
- What form of contraception are you using, if any? Do you use condoms?
- How do you feel about your current form of contraception? Discuss options if the patient wants to switch.
- Are there any risks or drug interactions of various forms of birth control with my CF medicines? If so, what are they?
- Should I be taking the lowest dose estrogen birth control pills?
*These questions can also be asked to a pharmacist specializing in complex care.
Contraception in CF
Multiple studies suggest rates of contraception use among women with CF are comparable to those of the general US population.[1] Rates range from 37% of young women ages 16-24 to 49% in a sample of adults.[2,3] Condoms and oral hormonal contraceptive pills are the most common forms of contraception used among women with CF. This correlates with rates for the general US female population.[4,5] Like others in the general population, women with CF have increased their use of long-acting reversible contraceptives (LARCs), like IUDs and implants, from 8% in 2016 to 27% in 2020.[3,5-7] Two reasons for this increase could be 1) the interaction of Orkambi™ with hormonal contraception and 2) the large number of people with CF who participate in clinical trials that require contraception use, and thus counseling by the research team.[8]
What kinds of contraception exist?
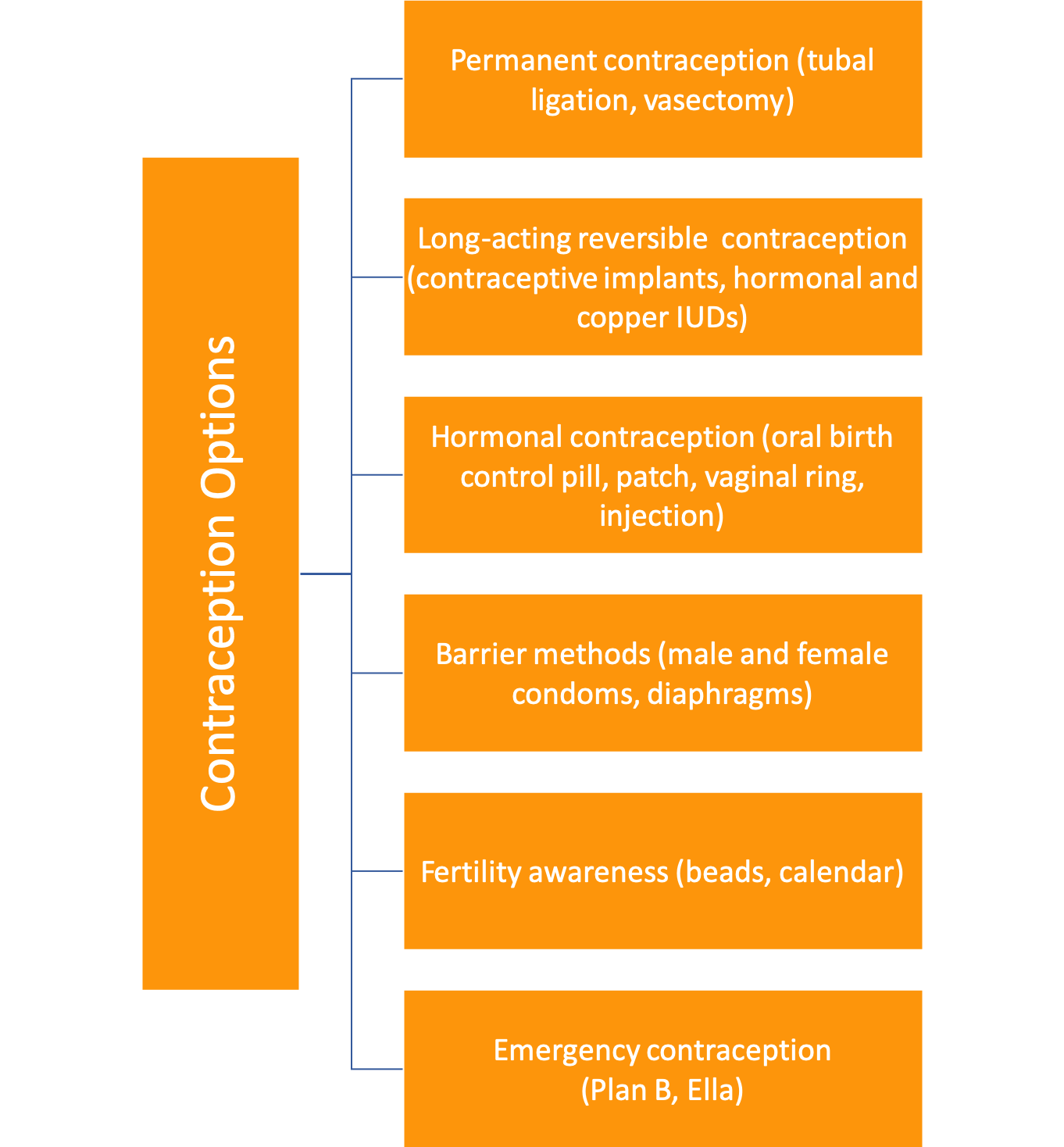
*For their part, male and female condoms can protect against STIs.
**To learn more about forms of birth control, please see the Resource Section at the end of this chapter.

Contraception Counseling
Females with CF receive their contraception care from a variety of providers. In a survey of women with CF (2018), Kazmerski et al. found that OB/GYNs prescribe or administer 66% of contraception to women with CF while CF clinics prescribe 7%. Primary care providers (PCPs) and other health care providers prescribe only a small percentage of them.
Researchers have shown that contraception counseling directly impacts contraception use, yet only 9% of young adults with CF have ever received such counseling from any source.[9] A study conducted in France (2019) found that introducing a gynecological consult into the CF clinic increased rates of contraceptive use –especially of LARCs– among women with CF.[10] Despite the fact that American CF clinicians believe contraception counseling is an important part of CF care, only a minority actually counsel their patients.[11] Barriers to patients and providers talking about contraception include: patient and provider discomfort, lack of time, and lack of training in contraception counseling.[11] Ideally, providers should give contraception counseling to patients on all medically eligible contraception options to allow them to make the appropriate choices for their lives.
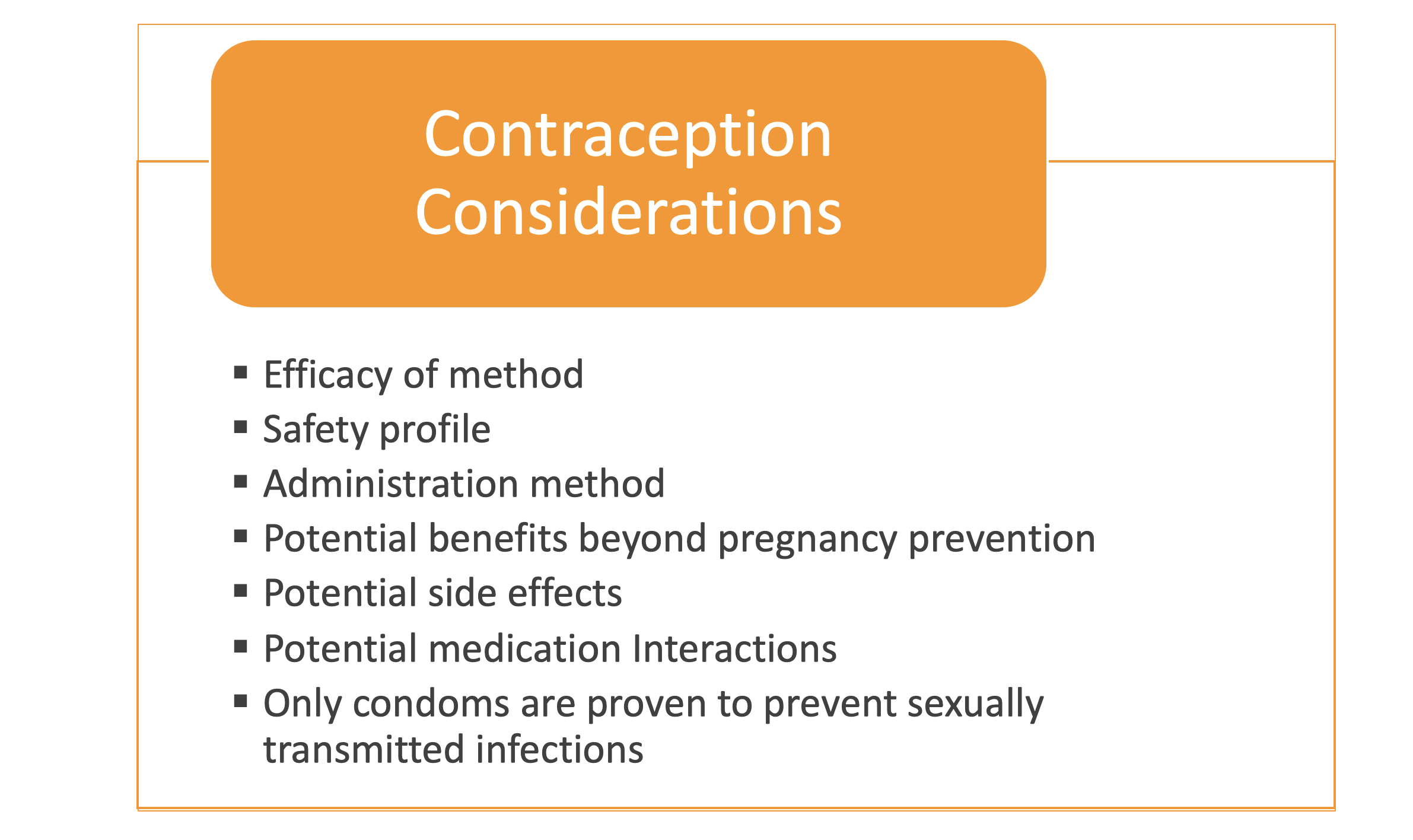
The Centers for Disease Control’s (CDC) US Medical Eligibility Criteria for Contraceptive Use (US-MEC) provides “recommendations for using specific contraceptive methods by women…who have certain…medical conditions.” CF was added to this chart in 2016 due to the advocacy of Dr. Emily Godfrey.[12] While safety data is currently limited for CF, the US-MEC table indicates “no risk” for most forms of contraception for CF (level 1). Only combined hormonal contraceptives are rated a Level 2, denoting that the “advantages generally outweigh theoretical or proven risks.” Neither level confers a contraindication to using a method; that is, patient preference can determine which method is used.[4] It is important to note that many people with CF have comorbidities such as transplant status, liver disease and diabetes that are also listed separately in the US-MEC guidelines. Please consult those specific guidelines and discuss which contraception options are safe and effective for each individual patient.
Myths about Contraception
Women with CF hold many misconceptions about contraceptive methods, which can reduce overall use [13]. LARCs are the most effective at preventing pregnancy of all methods, and females with CF typically experience minimal side effects [4]. The following modules clarify common myths about fertility and contraception.
Sources for this module include: 1. Shteinberg [14]; 2. Curtis [4]; Bedsider [15]; 3. Vertex [16]; 5. Curtis [4]; 6. Curtis [4].
Antibiotics make hormonal birth control less effective.
False
Most antibiotics women with CF routinely use do not interfere with hormonal birth control. Rifamycin antibiotics do interfere (see hormonal contraception section below for more information). Other medications, including some antifungals, may also interfere with hormonal birth control. When in doubt, consult your doctor or pharmacist.
You cannot take hormonal birth control with modulators.
Partly True
Orkambi™ reduces the effectiveness of hormonal contraceptives (e.g. oral contraceptives, patch and ring) in preventing pregnancy, so another form of birth control like IUDs or barrier methods should be used for birth control. All other modulators do not reduce the efficacy of hormonal contraceptives.
Women with CF are infertile.
False
Females with CF are not infertile. However, 35% experience subfertility, meaning it takes longer than one year to become pregnant. Sexually active women with CF are advised to use contraception unless they are actively trying to become pregnant.
[see the (In)Fertility chapter]
Intrauterine Devices (IUDs) are hormonal birth control.
Party True
While hormonal IUDs contain the progestin levonorgestrel, only small amounts of the hormone enter your blood. Hormonal IUDs do not contain estrogen. Typical CF medications do not make hormonal IUDs less effective at preventing pregnancy.
IUDs are not safe to use by women with CFRD.
False
All IUDs are safe for women who have CFRD, despite previous concerns that women who had diabetes would be at higher risk of pelvic inflammatory disease. However, the CDC states that this theoretical risk remains unproven.
Hormonal contraception is not safe to use if you have diabetes.
False
Combined hormonal contraception is safe to use in patients with diabetes. Studies have not found an increase in blood sugar glucose or insulin production in diabetic patients, except in cases of severe complications. The US-Medical Eligibility Criteria lists hormone-containing birth control as level 2 risk.
CF Specific Contraception Considerations
Hormonal Contraception: Unique Considerations for Women with CF
Although infrequent, estrogen-containing hormonal contraceptives may increase the risk for blood clots in some women, especially for those with a history of blood clots [4]. This is relevant because women with CF often have a higher incidence of blood clots due to liver issues, vitamin K deficiency, and the use of ports and PICC lines [17].
Combined hormonal contraception (CHC) usage can also limit treatment options for some CF infections. For example, Rifampin or rifabutin therapy, which is used to treat Multi-resistant staph aureus (MRSA) and Nontuberculosis Mycobacterium Avium Complex (MAC), reduces the effectiveness of hormonal contraception [18]. Providers should counsel patients using Rifampin to either use a second form of birth control (like condoms) or switch birth control methods to use a non-CHC method like an IUD (hormonal or copper), implant, or injection.
CF patients have an increased risk of developing osteopenia and osteoporosis due to decreased bone density. Because of these risks, women with CF are monitored biannually with a DEXA scan. Patients may lose bone density if they receive Depo-ProveraⓇ injections, but their bone density tends to improve after they discontinue it [19].

Reasons to use contraception beyond pregnancy prevention
Women with CF may benefit from using hormonal contraceptives to treat an underlying medical condition. Females with CF may have amenorrhea or irregular periods, for instance, because of low weight, recurrent lung infections, or stress [20]. Hormonal contraception can help regulate a woman’s period or eliminate it altogether, which may be particularly helpful for women with anemia or low iron. Some women with CF also experience fewer pulmonary exacerbations and a slower rate of lung function decline when using hormonal contraceptives. They may also need fewer courses of antibiotics [21,22] [See Hormones chapter].
Contraception Post-Transplant
Transplant patients have unique contraceptive challenges. Post-transplant medications containing mycophenolate, such as CellCeptⓇ, can reduce the effectiveness of hormonal birth control and can cause birth defects in a developing fetus. Because the effects can be so severe, the drug contains a Risk Evaluation and Mitigation Strategy (REMS) and black box warning from the FDA. As part of the REMS, the FDA requires patients and providers to sign a consent form prior to transplant in order to be treated with this class of drugs [23]. Since transplant medications interact with CHC, another method of birth control is recommended to prevent pregnancy throughout the period patient is using mycophenolate and at least six weeks after stopping [24]. If patient wants an IUD, it must be placed before transplant. In the first two years after transplant there is a small risk of infection [25]. Generally, the US-MEC recommends that a woman wait a minimum of 1-3 years post-transplant to consider pregnancy, during which time her contraceptive choices are especially important for her health and well-being [23].
CFTR Modulators and Contraception
While Orkambi ™ interacts with oral contraceptive pills, no other modulator does.
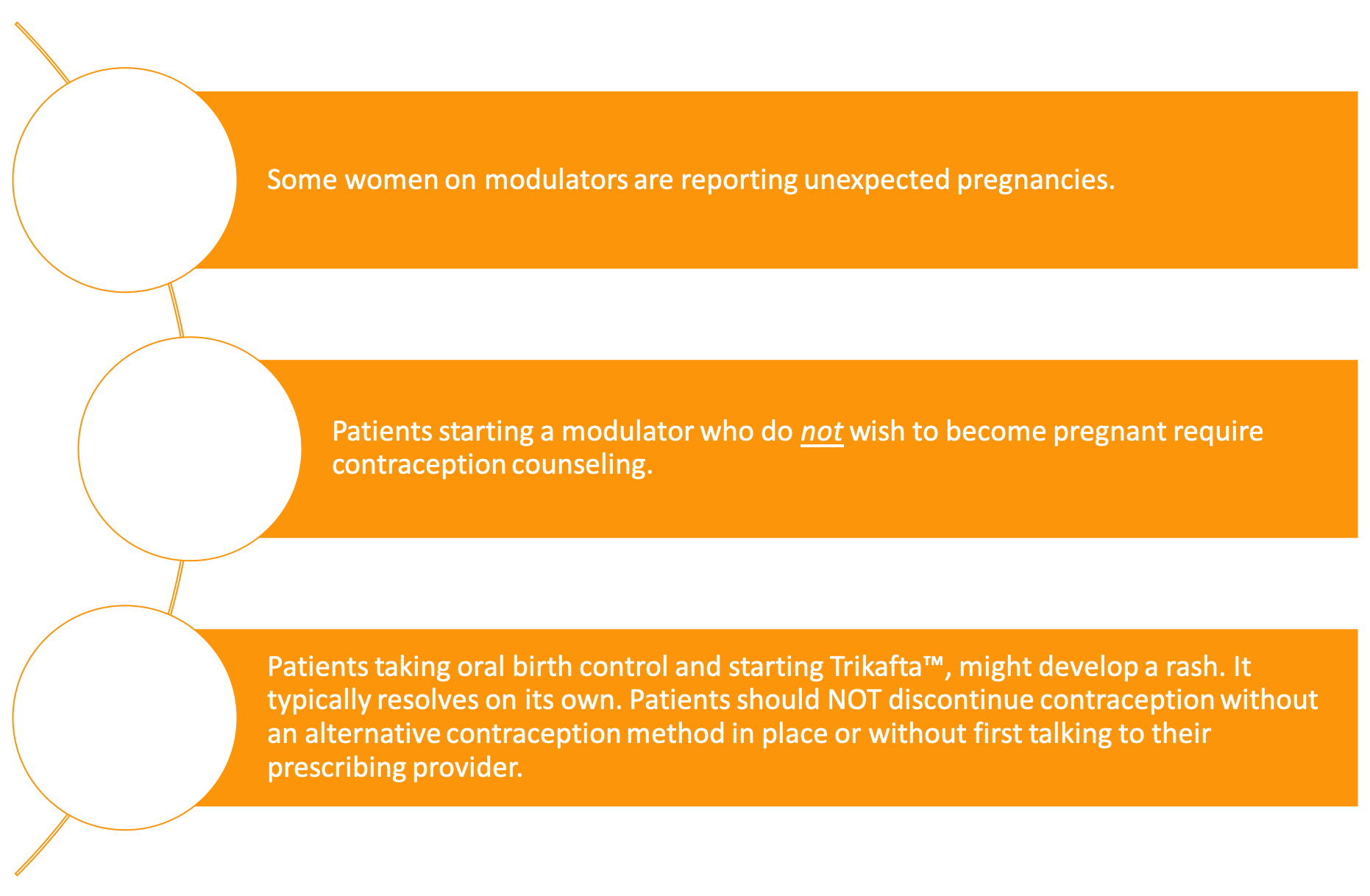
Many women with CF are reporting a “Trikafta baby boom.” Heltshe et al. recorded an increased pregnancy rate among women on KalydecoⓇ [26] While it is too soon to see if this result will hold for women on Trikafta™, there have been many anecdotal reports of women becoming pregnant shortly after initiating treatment. This highlights the need for providers to initiate conversations about contraception use and choices as well as pregnancy plans with their female patients before prescribing modulator therapy.
Hormonal contraceptives may play a role in rashes that are occuring in women taking Trikafta™ [27]. Should a woman on Trikafta™ experience a rash, it is important to select another form of birth control before stopping the current form to prevent an unplanned pregnancy. Condoms and other barrier methods can be used as a last-minute substitute. In these circumstances, it is best to consult a physician.
Psychosocial Implications
Many women with CF are often confused about whether they need contraception; they are also often uncertain about which option is best for them. From a young age, they face concerns about the possibility of being infertile. Consequently, many inaccurately believe they do not need to use contraception when they become sexually active [11].
Another concern many have is whether they are healthy enough to have a family. [see Family Building chapter]. It is therefore important for providers to understand that contraceptive use decisions can be fraught for many patients. Women with CF may decide – possibly at a young age – that they do not want to ever become pregnant. Providers should support these women in their decision. They can suggest LARCs over permanent forms of contraception, however, to preserve the woman’s option to make a different choice later in her life. In addition, preserving the sex organs can provide benefits for women with CF as they transition into menopause. [See the Menopause chapter].
What we want to know more about
There are major gaps in the research literature on contraception in CF. One way to aid contraception research in this area would be to add a question(s) about contraception use to the CF Foundation Patient Registry. Such a query would help provide long-term data about the efficacy, safety, lung health, and prescription interactions for each type of contraception and how it impacts women with CF. Godfrey et al. completed a pilot registry project that showed women with CF are amenable to CFF tracking such information [5].


Peer to Peer Advice
- There are many reasons to use hormonal birth control beyond preventing an unplanned pregnancy, like treating painful periods, irregular cycles, heavy flow, acne, etc.
- Who can you talk to? Find a professional with whom you feel comfortable discussing the topic of contraception.
- Assess and reassess your reproductive needs throughout your life.
- If you have side effects on a method of contraception, try another form. Talk to either your CF or women’s health provider about your options.
- Safe sex is important — does not mean safe sex! Only condoms prevent sexually transmitted infections.
- Pelvic exams are repeated regularly and are a great opportunity to discuss birth control options.
Resource Links
For comprehensive contraception choices charts see:
For medical safety information about contraception:
- CDC US-MEC safety charts
- CDC Medication summary
- There is even an app! Click here for Android and Apple
For more information about CF sexual and reproductive health:
- Center for Young Women’s Health Guide on Contraception
- Online Reproductive decision aid guide (Kazmerski; to be published 2021)
Works Cited
- Roe AH, Traxler S, Schreiber CA. Contraception in women with cystic fibrosis: A systematic review of the literature. Contraception. 2016;93(1):3-10. https://www.clinicalkey.es/playcontent/1-s2.0-S0010782415004801. doi: 10.1016/j.contraception.2015.07.007.
- Kazmerski TM, Sawicki GS, Miller E, et al. Sexual and reproductive health care utilization and preferences reported by young women with cystic fibrosis. Journal of cystic fibrosis. 2018;17(1):64-70. http://dx.doi.org/10.1016/j.jcf.2017.08.009. doi: 10.1016/j.jcf.2017.08.009.
- Roe AH, Traxler SA, Hadjiliadis D, Sammel MD, Schreiber CA. Contraceptive choices and preferences in a cohort of women with cystic fibrosis. Respiratory Medicine. 2016;121:1-3. https://www.clinicalkey.es/playcontent/1-s2.0-S0954611116302657. doi: 10.1016/j.rmed.2016.10.012.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR. Recommendations and reports. 2016;65(4):1-66. https://search.datacite.org/works/10.15585/mmwr.rr6504a1. doi: 10.15585/mmwr.rr6504a1.
- Godfrey EM, Mody S, Schwartz MR, et al. Contraceptive use among women with cystic fibrosis: A pilot study linking reproductive health questions to the cystic fibrosis foundation national patient registry. Contraception. 2020;101(6):420-426. http://dx.doi.org/10.1016/j.contraception.2020.02.006. doi: 10.1016/j.contraception.2020.02.006.
- Gatiss S, Mansour D, Doe S, Bourke S. Provision of contraception services and advice for women with cystic fibrosis. Journal of Family Planning and Reproductive Health Care. 2009;35(3):157-160. http://dx.doi.org/10.1783/147118909788708075. doi: 10.1783/147118909788708075.
- Plant BJ, Goss CH, Tonelli MR, McDonald G, Black RA, Aitken ML. Contraceptive practices in women with cystic fibrosis. Journal of Cystic Fibrosis. 2008;7(5):412-414. https://www.clinicalkey.es/playcontent/1-s2.0-S1569199308000271. doi: 10.1016/j.jcf.2008.03.001.
- Heltshe SL, Taylor-Cousar JL. Let’s talk about sex: Behaviors, experience and health care utilization in young women with CF. Journal of cystic fibrosis. 2018;17(1):5-6. http://dx.doi.org/10.1016/j.jcf.2017.11.006. doi: 10.1016/j.jcf.2017.11.006.
- Kazmerski TM, Sawicki GS, Miller E, et al. Sexual and reproductive health behaviors and experiences reported by young women with cystic fibrosis. Journal of Cystic Fibrosis. 2018;17(1):57-63. http://dx.doi.org/10.1016/j.jcf.2017.07.017. doi: 10.1016/j.jcf.2017.07.017.
- Rousset-Jablonski C, Reynaud Q, Perceval M, et al. Improvement in contraceptive coverage and gynecological care of adult women with cystic fibrosis following the implementation of an on-site gynecological consultation. Contraception (Stoneham). 2019;101(3):183-188. https://search.datacite.org/works/10.1016/j.contraception.2019.10.014. doi: 10.1016/j.contraception.2019.10.014.
- Kazmerski, Traci M., MD, MS, Borrero, Sonya, MD, MS, Sawicki, Gregory S., MD, MPH, et al. Provider attitudes and practices toward sexual and reproductive health care for young women with cystic fibrosis. Journal of Pediatric and Adolescent Gynecology. 2017;30(5):546-552. https://www.clinicalkey.es/playcontent/1-s2.0-S1083318817300220. doi: 10.1016/j.jpag.2017.01.009.
- Centers for Disease Control. US medical eligibility criteria (US MEC) for contraceptive use, 2016. https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html. Website. Accessed 9.10.2020.
- Traxler SA, Chavez V, Hadjiliadis D, Shea JA, Mollen C, Schreiber CA. Fertility considerations and attitudes about family planning among women with cystic fibrosis. Contraception (Stoneham). 2019;100(3):228-233. http://dx.doi.org/10.1016/j.contraception.2019.05.005. doi: 10.1016/j.contraception.2019.05.005.
- Shteinberg M, Lulu AB, Downey DG, et al. Failure to conceive in women with CF is associated with pancreatic insufficiency and advancing age. Journal of cystic fibrosis. 2019;18(4):525-529. https://search.datacite.org/works/10.1016/j.jcf.2018.10.009. doi: 10.1016/j.jcf.2018.10.009.
- Bedsider. Which medication can mess with birth control? https://www.bedsider.org/features/294-which-medications-can-mess-with-birth-control. Accessed 1.2020.
- OrkambiⓇ [package insert]. Boston, MA: Vertex Pharmaceuticals; Updated 2015. Accessed 1.2020.
- Blower K, DO, Seifi A, MD, Michalek J, PhD, Keyt H, MD. Incidence of VTE in patients with cystic fibrosis: Are they high risk? Chest. 2016;150(4):1135A. https://www.clinicalkey.es/playcontent/1-s2.0-S0012369216574443. doi: 10.1016/j.chest.2016.08.1245.
- Floto RA, Olivier KN, Saiman L, et al. US cystic fibrosis foundation and european cystic fibrosis society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(Suppl 1):i1-i22. http://dx.doi.org/10.1136/thoraxjnl-2015-207360. doi: 10.1136/thoraxjnl-2015-207360.
- Depo provera [drug insert]. NY, NY: Pfizer inc.; Updated 2010. Accessed 1.2020.
- Hughan KS, Daley T, Rayas MS, Kelly A, Roe A. Female reproductive health in cystic fibrosis. Journal of cystic fibrosis. 2019;18:S95-S104. https://search.datacite.org/works/10.1016/j.jcf.2019.08.024. doi: 10.1016/j.jcf.2019.08.024.
- Perrissin-Fabert M, Stheneur C, Veilleux-Lemieux M, et al. Hormonal contraception effects on pulmonary function in adolescents with cystic fibrosis. Journal of pediatric & adolescent gynecology. 2020. http://dx.doi.org/10.1016/j.jpag.2020.07.014. doi: 10.1016/j.jpag.2020.07.014.
- Chotirmall SH, Smith SG, Gunaratnam C, et al. Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. The New England journal of medicine. 2012;366(21):1978-1986. https://search.datacite.org/works/10.1056/nejmoa1106126. doi: 10.1056/nejmoa1106126.
- FDA. Mycophenolate risk evaluation mediation strategy. US food and drug administration website. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-mycophenolate Updated 2015. Accessed 1.2020.
- CellCeptⓇ [package insert]. Nutley, New Jersey: Roche Laboratories inc.; Updated 2018. Accessed 1.2020.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR. Recommendations and reports. 2016;65(3):1-103. https://search.datacite.org/works/10.15585/mmwr.rr6503a1. doi: 10.15585/mmwr.rr6503a1.
- Heltshe SL, Godfrey EM, Josephy T, Aitken ML, Taylor-Cousar JL. Pregnancy among cystic fibrosis women in the era of CFTR modulators. Journal of Cystic Fibrosis. 2017;16(6):687-694. https://www.clinicalkey.es/playcontent/1-s2.0-S1569199317300152. doi: 10.1016/j.jcf.2017.01.008.
- Trikafta [package insert]. Boston, MA: Vertex Pharmaceuticals; Updated 2019. Accessed 1.2020.
Free Printable PDF Download
Want a free printable PDF download of this section for your use in clinic? Just give us your name and email address below to get your download link. This will not add you to our email list.
